The Miniaturisation of Cardiac Care – How Smaller Means Safer
Posted on: in Blog
According to the World Health Organisation, cardiovascular disease accounts for an estimated 17.9 million worldwide deaths a year and it is the number 1 cause of death globally. There are two types of cardiac active implanted devices, designed for patients with heart rhythm disorders and heart failure. These are life critical such as pacemakers, implantable cardioverter defibrillator (ICD) biventricular pacemakers which maintain the pace of the heart; and cardiac monitoring sensors which monitor heart rhythm and other cardiac parameters. The latter have sensors placed inside miniature devices that monitor vital cardiac signs such as pulmonary blood pressure which can detect high pressure before a heart attack.
Over a million cardiac pacemakers are implanted every year making it one of the most common types of heart surgery. In 2018, the global pacemaker market was worth $4.8 billion, forecasted to reach $5.3 billion by 2026. However the cost of pacemaker surgery is not just implanting the pacemaker, it’s also the cost of explanting the device (often more expensive than implantation), should there be any problem e.g. pain, infection, battery renewal as well as the cost to the patient’s health from further invasive surgery.
Present day pacemakers are placed in the chest with leads into the heart. Next-generation leadless pacemakers devices will be placed directly into the heart with no leads required meaning a less intrusive operation and therefore less trauma to the patient. By energy harvesting from the beating of the heart using a piezo-electric technology, such as that developed by Cairdac, miniature batteries can be integrated into the implant as a backup power source to replace the current larger non-rechargeable battery a pacemaker requires due to its high energy usage.
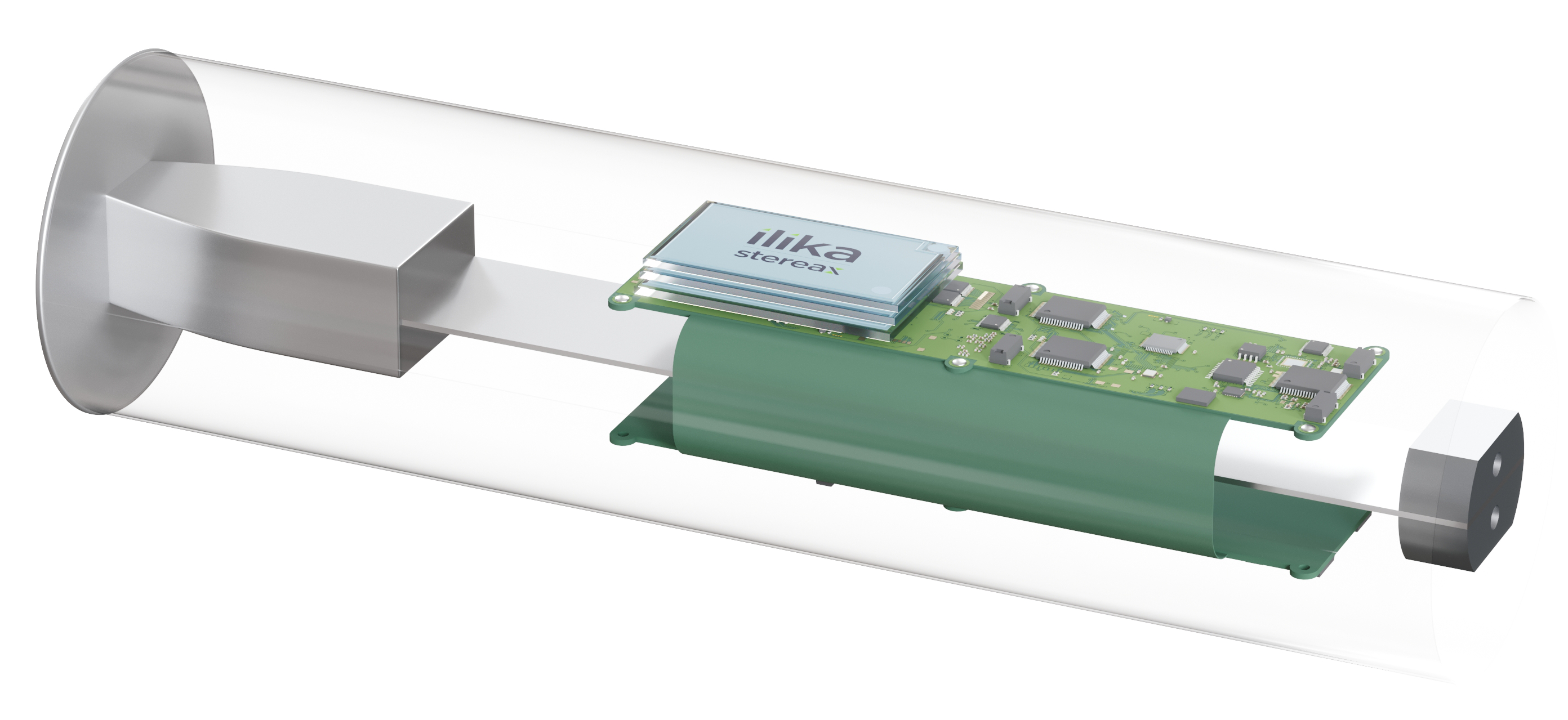
Cardiac monitoring devices have sensors within that continuously monitor the heart rhythm (loop recorders) or blood pressure and require less power than pacing devices. It’s also a growing market, valued at $410.4 million in 2016 and forecast to achieve $678.3 million in 2023. As in other areas of MedTech, product designers of these next-generation cardiac devices are embracing miniaturisation. The smaller the device, the less invasive the procedure to implant it and the closer it can placed be to the target organ. They are so small they will be inserted via a catheter and placed in an artery, for example in the lungs.

In addition to patient benefits, there are many financial benefits to these new cardiac implantable devices making them appealing to healthcare providers and insurers alike:
- The devices are so small they can be inserted via a catheter meaning less invasive surgery that will require less medical staff.
- As there are no leads from the pacemaker to the heart, there’s no need for costly surgery to remove them when they stop working properly – a situation made worse if they’ve been in place for many years as they’ll be surrounded by scar tissue making their extraction more difficult and dangerous.
- Reduced surgery means reduced bed days and less medication costs.
- Lesser risk of complications leading to a costly explantation
- Reduced costs whilst improved patient experience is more favoured by healthcare insurers.
In addition, the batteries in most traditional pacemakers last for 6 to 10 years when they need to be replaced, resulting in more costly surgery as explanting the device is often more costly that implanting it. The patient at this point, is likely to be in their later years so the surgery become even more high risk.
However, for these devices to reach their full potential, their design can’t be dictated by the size and functionality of the battery available. Battery technology must be designed to enable the device to work at its best. And that’s what we’ve done at Ilika, working closely with next-generation product designers to develop our Stereax miniature solid state batteries. They are less than 1mm thin, not only customisable in shape and form but can also be stacked to increase energy. They are long lasting – up to 10 years, can be wirelessly charged and can also harvest energy from the vibration of the heart. The device is powered by the battery which in turn is powered by the heart the device is keeping going.
Being solid state they don’t have any toxic electrolyte to leak and also don’t require a metallic can to contain this toxic substance enabling them to have a footprint of only a few mm². In addition, they have a rate capability up to 10-20C, yielding mA of possible peak current and rapid recharges.

Our new Stereax M300 is designed for medical implants. It is:
- · 5.6 x 3.6 mm
- · 0.9 mm thick
- · 300 mAh
- · 3 mA standard current
- · Thousands of cycles
After release of the M300 we’ll continue working on developing our solid state batteries to increase energy density, targeting 200 Wh/L.
If you’d like to know more about how our mm-scale solid state batteries can power your next-generation cardiac implant contact us now at info@ilika.com
